Physical chemistry - Calculations of enthalpy - Examples and exercises
1) Using the data given on the table below:
| Bond | Bond energy (kJ/mol) |
| H-H | 436 |
| Cl-Cl | 243 |
| H-Cl | 432 |
Calculate ΔH for the reaction:
H2(g) + Cl2(g) → 2HCl(g)
in kJ per mol of HCl(g), which equals:
a) –92.5 b) –185 c) –247 d) +185 e) +92.5
Answer:
We calculate the energy input and output of the reaction, as in here.
Input (bonds to be broken)
H-H + Cl-Cl = 436+243 = 679
Output (bonds to be formed)
2 H-Cl = 2* 432 = 864
The calculation shows that more energy is released than is needed to initiate the reaction. That means that the reaction is exothermic and so ΔH is negative.
To get the number we subtract intput from output: 679-864 = -185
That is to form 2 mol of HCl (see equation). For 1 mol it is half of that.
Answer:
b) –92.5
2) When a salt is dissolved in water, thermal processes may occur, so that the water may get cooled or heated up.
What is the heat change (enthalpy of solution) that occurs when 1 mol of sodium chloride dissolves in water?
Use the data on the diagram below.
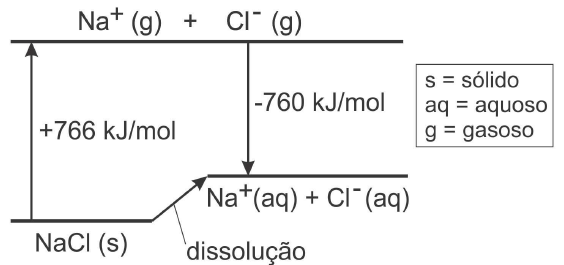
a) it is very exothermic, involving around 1000 kJ.
b) it is very endothermic, involving around1000 kJ.
c) no heat change
d) it is slightly exothermic, involving less than 10 kJ.
e) it is slightly endothermic, involving less than 10 kJ.
Answer:
The diagram shows explicitly the energy transformations , the vaporization of NaCl and the change from the gas phase to solution in water, of the ions Na+ and Cl-.
Applying Hess Law, it is possible to calculate the energy change asked in the problem.
The diagram shows that 766 kJ/mol are required to vaporise solid NaCl. The vaporised ions are shown in a level above that for the solid . The process is endothermic, because heating is required for this change to occur.
The level of the ions in solution is also above that of the solid. It means that for the solid to dissolve, energy is required. Energy is removed from the water so that the process is endothermic. The water gets cooler after the solution of the NaCl.
The difference between the level of the soild , and the level of the ions in solution, can be easily calculated, just by looking at the diagram.
It is 6 kJ/mol.
So, the answer is :
e) it is slightly endothermic, involving less than 10 kJ.
3) The bombardier-beetle atacks its predators with a hot and nasty solution of benzoquinone. This is generated from hydroquinone and oxygen, which is liberated from hydrogen peroxide by enzimes in the "reactor vessel" inside the insect.
This reaction, the oxidation of hydroquinone, is highly exothermic:
C6H4 (OH)2(aq) + H2O2(aq) →C6H4O2 (aq) + 2 H2O(l)
Calculate the heat involved in this reaction, which can be calculated from the following reactions:
1) C6H4 (OH)2(aq) → C6H4O2 (aq) + H2(g) ΔH = 177 kJ/mol
2) H2O(l) + 1/2 O2 (g) → H2O2(aq) ΔH = 95 kJ/mol
3) H2O(l) → 1/2 O2 (g) + H2 (g) ΔH = 286 kJ/mol
a) -558 kJ mol-1
b) -204 kJ mol-1
c) +177 kJ mol-1
d) +558 kJ mol-1
e) +585 kJ mol-1
Answer:
The equations can be manipulated like in algebra (Hess's Law), as shown in here. If one is reversed, the sign of ΔH is also reversed.
The trick is to add or subtract the equations given in order to assemble the one we are interested. The corresponding ΔH's will also be added / subtracted so that we get to the answer we want.
This is done by trial and error and it requires a little bit of practice.
Inverting reactions 2 and 3 and adding them to reaction 1, we obtain the equation we want. Terms that appear on both sides of the equation can be eliminated, like in ordinary algebra.
So, the answer is 177 - 95 - 286 = -204.
b) -204 kJ mol-1
Ways of calculating enthalpy of reaction:
3) Using enthalpies of formation
