Physics > Thermal physics > Introduction - Particle model
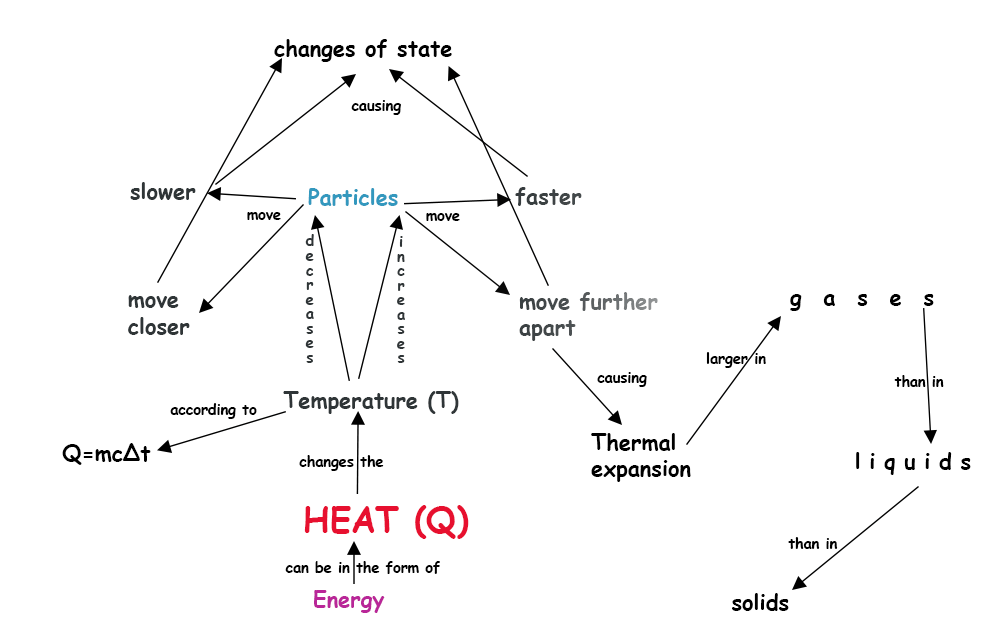
The particle model enables us to understand a range of natural phenomena. It is about submicroscopic particles, like atoms and molecules that are jiggling constantly according to the temperature. The higher the temperature, the faster the jiggle. This motion is permanent and persists even at the absolute zero temperature which is 0 K or –273 °C .
The particle model enables us to understand the temperature, phase transitions (like boiling or melting) , gas pressure, the heating of gases under compression and the cooling when undergoing expansion, among many others.
Great ideas
The great ideas PARTICLE and ENERGY are the key to understand this topic. Everything in the world (and in the whole universe) is made of particles like atoms and molecules. They form all the materials. Energy, on the other hand, is what makes things happen. Without energy there is no life, np movement, nothing. Imagine a planet without any available energy: it would be completely dead.
Kinetic theory
If everything is made of particles and we can understand and predict how they behave, by using Newton's laws of motion, we can (at least in principle) understand everything!
The application of the particle model and Newton laws to the study of gases is called kinetic theory, and it is very successful. It explains pressure and many other properties. It is used to deduce the gas equation PV=cte.
The GCSE syllabus only mentions PV=cte but it doesn´t hurt to look at the complete equation:
PV = nRT
which is rediced to the previous one, PV=cte, when the number of mols of particles (n) and the temperature are constant. The number R (the gas constant) is always constant, because it is a constant.
It is curious the fact that there are universal constants. What if they had other values? Tis issue is discussed here.
